Acids are dangerous, wondrous things. They help us dissolve food, make products, clean things, just about everything. But the strongest one could dissolve pretty much anything in no time, including your body.
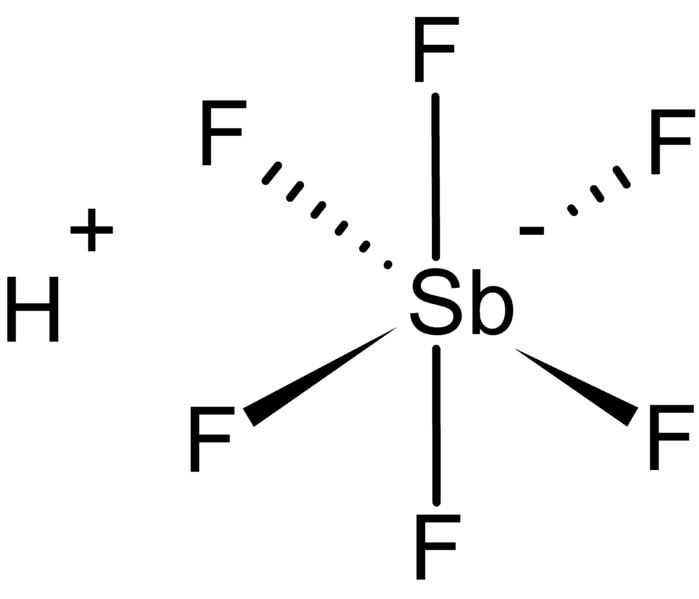
Fluoroantimonic acid is the strongest super-acid known in existence. It is 20 quintillion times more acidic than 100% sulfuric acid, and it can dissolve glass plus a host of other substances. This particular acid is used as a catalyst in chemical reactions for biochemistry, gasoline production, and the making of synthetic materials. It’s made up of antimony, fluorine, and hydrogen. The weak bond between the hydrogen ion and fluorine is what makes this acid so destructive and extremely acidic. Once fluoroantimonic acid loses a proton, it begins to tear electrons from atoms.
The strength of this acid is remarkable, so then, how is it stored? If it were held in a glass bottle, it would dissolve the bottle and the hand you were holding it with. The acid is only able to be held by a thing that we know and love when cooking in a pan where we don’t want the food to stick. It’s Teflon, or polytetrafluoroethylene. It has the strongest single bond in organic chemistry between carbon and fluorine. The result is a very strong chemical structure. The containers containing the super-acid have a coating of Teflon so the acid won’t eat a hole through the container, the floor, your hand, etc., etc.
Sources: Real Clear Science, Chemistry Answers