It seems to go against any preconceived notion that hot water freezes faster than cold water under certain conditions, and until now, scientists have been unable to explain why it does happen. Now they have a reason.
It’s a fact that hot water, under certain conditions, can freeze faster than cold water. It was first discovered by a Tanzanian student in 1963, named Erasto Mpemba. Mpemba noticed something peculiar while in a cooking class he was taking. He observed that a hot ice cream mix froze faster than a cold mix. He later posed the question of why to Dr. Denis Osbourne, a physicist, who later tested Mpemba’s observation. He found that it was true, and the phenomenon became known as the Mpemba effect.
Mpemba wasn’t the first to notice this happening, however. Aristotle observed a similar action in the 4th century B.C., as did Rene Descartes and Sir Francis Bacon. But Mpemba, with Dr. Osbourne’s help, wrote the first published scientific paper in 1969 on the effect. Even though the Mpemba effect was clearly shown, scientists were baffled why it happened and under what circumstances needed to be present for it to take place.
Many theories were proposed on what would seem like an easy answer. One theory proposed that because of the faster evaporation of hot water, the volume left to freeze was reduced. Another theory pointed to a frost layer on cold water which insulated it from freezing faster, and yet another said it must have something to do with different solutes contained in the water that are removed with heating.
As science sometimes is, the Mpemba effect doesn’t occur all the time. Often cold water will freeze faster than hot water, but the Mpemba effect still occurs regularly under certain conditions. Up until this time, there were only theories on why it happened. It wasn’t until a group of physicists recently came upon an answer to the seemingly easy problem.
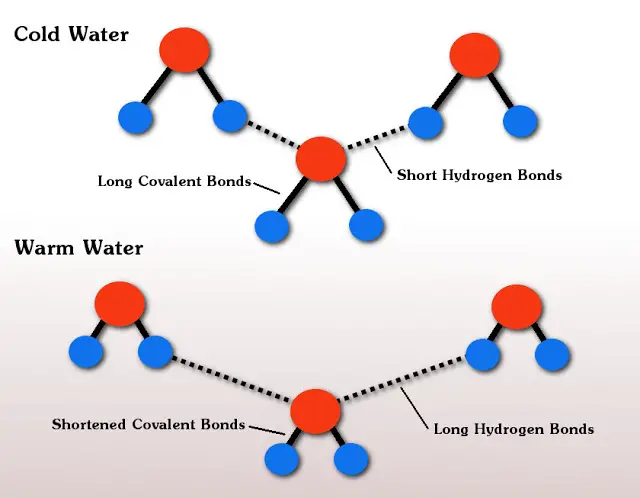
Physicists from Nanyang Technological University in Singapore discovered that the reason for the Mpemba effect lies in the chemical bonds that hold the water molecules together. A water molecule has one oxygen atom and two hydrogen atoms that have a covalent bond (a chemical bond that involves the sharing of electrons between atoms). The water molecules are also held together to other water molecules by weaker hydrogen bonds. This happens when a hydrogen atom is sitting close to an oxygen atom of another water molecule. It is believed that these weaker bonds are what essentially cause the Mpemba effect.
As you can see in the figure above, before the water is heated, water molecules are close together and “push” against each other. The hydrogen covalent bonds of each individual water molecule are then stretched, which makes them store energy. When the water is heated, the water becomes less dense and the water molecules begin to move apart. This causes the weaker bonds between molecules to now stretch.
This stretching, or the move apart between water molecules, allows the covalent bonds to relax and give up their energy or their heat. In theory, this results in cooling, so the warm water cools faster than the cold water, and under all the right conditions, allows it to freeze faster than cold water would.
It may be easier to think of those bonds like a rubber band. When the rubber band is pulled back it stores energy (which would be heat), when released, that energy is given up and transferred to the environment. In this case, when freezing cold water, the energy in those covalent bonds is never released as it would be in warm water.
If your mind is spinning, take heart that scientists continue to argue about the Mpemba effect. It’s rather amazing that something that seems like it could be discovered by a simple method has perplexed minds for centuries.