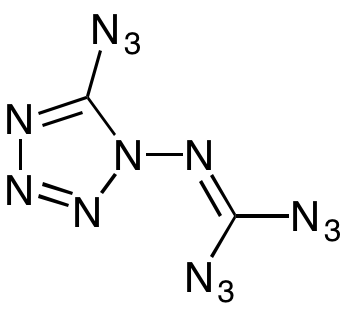
The most explosive chemical in the world is a high-nitrogen energetic material called 1-Diazidocarbamoyl-5-azidotetrazole or C2N14 and informally called “azidoazide azide.” C2N14 has 14 nitrogen and two carbon atoms, and it is so highly reactive that it will explode in about every situation, even if it’s not even touched.
When German researchers were daring enough to try to work with it in 2011, they found that it exploded when it was touched, moved, or put in a solution. It even exploded when they tried to get an infrared spectrum of it in what would seem to be a safe test but was not.
C2N14 is incredibly endothermic, which means the chemical reaction absorbs heat, and it also has bonds that are tremendously unstable. Its 14 high-energy nitrogen bonds are what make it so incredibly explosive. This also leads to why the compound is so unstable and why there is no way to measure it. Any movement or test applied as a solid or in a solution results in an explosion. The only test researchers could get was an x-ray of the crystal structure.
Only very tiny amounts of the material have been made experimentally, and it doesn’t have any known application other than it explodes. This is a substance scientists may never feel the need to work with again, nor would they want to try.
Some Other Nasty Chemicals
Another chemical that tries to outdo C2N14 is chlorine trifluoride. This chemical is highly corrosive and makes pretty much everything, even things considered non-flammable or fireproof, catch fire or explode. These fires also can’t be put out.
The only way to store this chemical is in containers made of nickel, copper, iron, or steel after they’ve been treated with fluorine gas, which creates a thin layer in the container, protecting it from being oxidized.
In the 1950s, a ton of chlorine trifluoride was being moved in a steel container that had been cooled by dry ice to make the chemical safer to handle, but the dry ice made the container brittle. The chemical spilled on the floor and burned its way through a foot (30 centimeters) of concrete and three feet (90 centimeters) of gravel. The resulting fumes corroded everything it came in contact with. In the 1990s, it found a use in the semiconductor industry cleaning tool chambers.
And lastly, there is dimethyl cadmium, not so much for its explosive or corrosive ability but more for how incredibly toxic it is. If this chemical is left out, it can either ignite, which gives off poisonous cadmium oxide smoke, or it can react with oxygen and form a dimethyl cadmium peroxide crust, which can then explode. A few micrograms of dimethyl cadmium vapor are extremely toxic and can affect the liver, kidneys, heart, and lungs. And just to add to that, cadmium is also carcinogenic.
Sources: Science.org (1), Chemistry World, Discovery (1), American Chemical Society, Scientific American, Wiley Online Library, Discovery (2), Air Products, Science.org (2)